CSP-270
Meibomian Gland Dysfunction
CSP-270
Inflammation and Pain after Ocular Surgery
CSP-270
Uveitis
OT-270
Diabetic Macular Edema
CSP-270 cream contains a novel selective glucocorticoid receptor agonist (GW870086) in development for the treatment of Meibomian Gland Dysfunction (MGD). Uniquely the proprietary cream is applied once daily to the outside of the eyelid where it forms a depot that delivers drug to the surface of the eye every time the patient blinks.
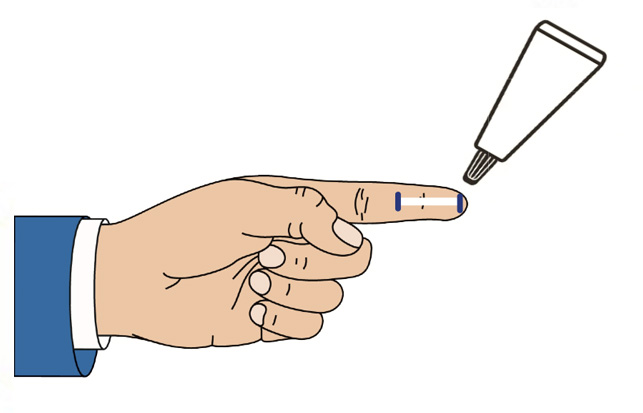
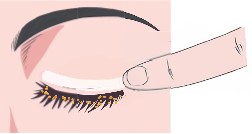
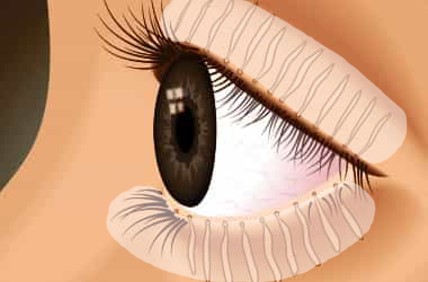
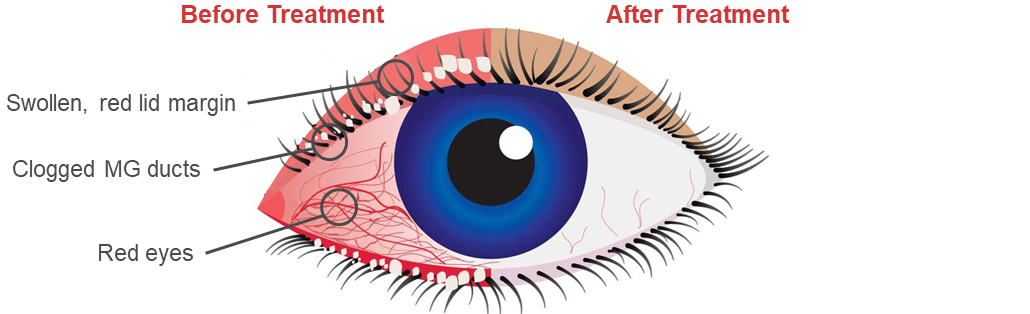
This novel route of administration offers competitive advantages over routes of administration such as eye drops, increasing the concentration of drug delivered to ocular structures and eliminating the risk of wash-out due to tearing.
CSP-270 has achieved clinical proof-of-concept in patients with MGD. In phase 2 clinical studies, CSP-270 showed a 30% improvement in VAS of ocular discomfort after 3 weeks treatment. The product, which is phase 3 ready, is safe and well tolerated with no ocular TEAEs and no increases in intraocular pressure (IOP) reported.
The prevalence of MGD is expected to grow dramatically owing largely to an ageing population and increasing use of digital screens. There is high unmet need for a treatment with a faster onset of action. Convenience of dosing, rapid onset of action and lack of IOP increase are major strengths of the CSP-270 cream, that have been estimated to translate to peak revenues opportunities of over $2bn.
